 This product, when used as directed, produces an industrial bleach that can cause serious harm.
This product, when used as directed, produces an industrial bleach that can cause serious harm.
Swallowing doses of this bleach, such as those recommended in the labeling can cause nausea, vomiting, diarrhea, and symptoms of severe dehydration. Continue reading Consumer Alert: Miracle Mineral Supplement aka MMS →
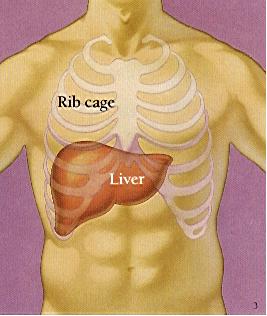 Silymarin (Silybium marianum), an extract of milk thistle, is often used to treat chronic liver disease.
Silymarin (Silybium marianum), an extract of milk thistle, is often used to treat chronic liver disease.
Researchers with the Silymarin in the NASH and C Hepatitis (SyNCH) Study Group studied its effect when combined with interferon. Continue reading Milk thistle fails in study of Hep C treatment →
 FDA is warning consumers that Reumofan Plus, marketed as a natural dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful.
FDA is warning consumers that Reumofan Plus, marketed as a natural dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful.
Here’s what we know. Continue reading Consumer alert: Reumofan Plus →
 Man Up Now claims to be “herbal” and “all natural.” Consumers may mistakenly assume the product is harmless and poses no health risk. One consumer below thinks it no big deal.
Man Up Now claims to be “herbal” and “all natural.” Consumers may mistakenly assume the product is harmless and poses no health risk. One consumer below thinks it no big deal.
Consumers who have Man Up Now capsules should stop using them, because the FDA determined it contains sulfoaildenafil, a chemical similar to sildenafil, and the active ingredient in Viagra. Continue reading Consumer Alert: Man Up Now capsules →
 The FDA tells us that Wholistic Herbs Inc. is recalling all of its “Koff & Kold” spray with herbal extract and “Kold Sore” spray with liquid sea mineral. Continue reading Consumer Alert: “Koff & Kold” and “Kold Sore” →
The FDA tells us that Wholistic Herbs Inc. is recalling all of its “Koff & Kold” spray with herbal extract and “Kold Sore” spray with liquid sea mineral. Continue reading Consumer Alert: “Koff & Kold” and “Kold Sore” →
 According to the FDA, RegenArouse (Lot 130100) contains tadalafil (Cialis), an FDA-approved drug used as treatment for male erectile dysfunction, making this product an unapproved new drug. Continue reading Consumer Alert: RegenArouse →
According to the FDA, RegenArouse (Lot 130100) contains tadalafil (Cialis), an FDA-approved drug used as treatment for male erectile dysfunction, making this product an unapproved new drug. Continue reading Consumer Alert: RegenArouse →
 This is the first time FDA has taken legal action against a dietary supplement manufacturer (ATF Fitness Products) of this size for failure to comply with the dietary supplement current Good Manufacturing Practice (cGMP) regulations. Continue reading Consumer Alert: FDA takes legal action against ATF Fitness Products Inc. →
This is the first time FDA has taken legal action against a dietary supplement manufacturer (ATF Fitness Products) of this size for failure to comply with the dietary supplement current Good Manufacturing Practice (cGMP) regulations. Continue reading Consumer Alert: FDA takes legal action against ATF Fitness Products Inc. →
 FDA has recalled Pentrexyl Forte Natural because the packaging is believed to be misleading — causing it to be confused with an antibiotic.
FDA has recalled Pentrexyl Forte Natural because the packaging is believed to be misleading — causing it to be confused with an antibiotic.
Continue reading Consumer Alert: Pentrexyl Forte Natural →
 The FDA and the Federal Trade Commission (FTC) today announced a joint effort to remove products from the market that make unproven claims to treat, cure, and prevent sexually transmitted diseases (STDs).
The FDA and the Federal Trade Commission (FTC) today announced a joint effort to remove products from the market that make unproven claims to treat, cure, and prevent sexually transmitted diseases (STDs).
Among the products targeted are Medavir, Herpaflor, Viruxo, C-Cure, and Never An Outbreak. Continue reading Consumer Alert: Fraudulent STD treatments →
 FDA is warning companies to stop making MRSA claims.
FDA is warning companies to stop making MRSA claims.
Methicillin-resistant Staphylococcus aureus (MRSA) bacteria can cause an infection that’s highly resistant to some antibiotics. MRSA cause infection after they enter the body through a cut or sore.
The US Food and Drug Administration has issued 4 warning letters to companies that manufacture and market over-the-counter (OTC) drug products (including hand sanitizers) that claim to prevent infection by MRSA. Continue reading FDA warns companies to stop making MRSA claims →
 FDA lab analysis of X-Hero found the product contains sulfosildenafil, the analogue of the active ingredient of an FDA-approved drug (sildenafil, Viagra, Revatio) used to treat erectile dysfunction.
FDA lab analysis of X-Hero found the product contains sulfosildenafil, the analogue of the active ingredient of an FDA-approved drug (sildenafil, Viagra, Revatio) used to treat erectile dysfunction.
In addition, FDA analysis of Male Enhancer found it contains tadalafil (Cialis, Adcirca), the active ingredient of an FDA-approved drug used to treat erectile dysfunction. Continue reading Consumer Alert: X-Hero and Male Enhancer →
 Being overweight after breast cancer treatment may increase a woman’s risk for recurrent disease.
Being overweight after breast cancer treatment may increase a woman’s risk for recurrent disease.
Researchers at the University of Arizona, in Tucson found that there are benefits from drinking green tea, but weight loss isn’t one of them. Continue reading Effects of green tea in obese breast cancer survivors →
 FDA has received reports of adverse events associated with the use of Fruta Planta, including several cardiac events and one death.
FDA has received reports of adverse events associated with the use of Fruta Planta, including several cardiac events and one death.
In addition, FDA testing revealed that certain lots of RockHard Weekend and Pandor contain an analogue of sildenafil, an FDA-approved drug used as treatment for male erectile dysfunction. Continue reading Consumer Alert: Rockhard Weekend, Pandora, Fruta Planta →
 There are “significant public health problems posed by products that are marketed as dietary supplements but that contain the same active ingredients as FDA-approved drugs… that do not qualify as dietary ingredients.” Continue reading FDA makes its position clear on dietary supplements →
There are “significant public health problems posed by products that are marketed as dietary supplements but that contain the same active ingredients as FDA-approved drugs… that do not qualify as dietary ingredients.” Continue reading FDA makes its position clear on dietary supplements →
 The FDA tells us that Vigor-25, a product marketed as a natural dietary supplement to enhance male sexual performance, should not be purchased or used because it contains sildenafil, the active ingredient in the prescription drug Viagra.
The FDA tells us that Vigor-25, a product marketed as a natural dietary supplement to enhance male sexual performance, should not be purchased or used because it contains sildenafil, the active ingredient in the prescription drug Viagra.
The FDA is investigating the reported death of a 26-year old man, possibly associated with the use of Vigor-25. Continue reading Consumer Alert: Vigor-25 →
 8 companies of over-the-counter (OTC) chelation products were told that they are selling unapproved drugs and devices and are violating federal law with respect to unproven claims about these products.
8 companies of over-the-counter (OTC) chelation products were told that they are selling unapproved drugs and devices and are violating federal law with respect to unproven claims about these products.
These products purport to treat a range of diseases by removing toxic metals from the body. Some also claim to treat autism spectrum disorder, cardiovascular diseases, Parkinson’s disease, Alzheimer’s disease, macular degeneration, and other serious conditions. Continue reading Consumer Alert: FDA warns about OTC chelation products →
 The FDA is taking action to prevent Rising Sun Health and The Center for Complimentary and Alternative Health of Livingston, Montana, from manufacturing and selling unapproved new drugs and adulterated or misbranded dietary supplements in violation of the law.
The FDA is taking action to prevent Rising Sun Health and The Center for Complimentary and Alternative Health of Livingston, Montana, from manufacturing and selling unapproved new drugs and adulterated or misbranded dietary supplements in violation of the law.
Rising Sun manufactured and distributed a variety of unapproved new drugs under names such as Black Salve, Cancema, and Can-Support. Continue reading Consumer Alert: Rising Sun Health →
 So good they had to name it “slimming” twice.
So good they had to name it “slimming” twice.
Unfortunately, Slimming Beauty contains the active pharmaceutical ingredient sibutramine (Meridia), a stimulant. And the marketer, Beautiful Health Inc., formerly LL Health and Beauty, failed to list it among the ingredients on the label. Continue reading Consumer Alert: Slimming Beauty Bitter Orange Slimming Capsules →
 Aromatase inhibitors are a class of drugs used to treat breast cancer and ovarian cancer in postmenopausal women.
Aromatase inhibitors are a class of drugs used to treat breast cancer and ovarian cancer in postmenopausal women.
Now, the FDA is recalling the following supplements that illegally contain these drugs. Continue reading Consumer Alert: Aromatase inhibitors in dietary supplements →
 It’s labeled as “100% natural” and consumers may mistakenly assume the product is harmless and poses no health risk.
It’s labeled as “100% natural” and consumers may mistakenly assume the product is harmless and poses no health risk.
The FDA says, Not so fast, all you erectilely challenged people out there. Continue reading Consumer Alert: TimeOut →
Complementary and Alternative Medicine: Fair, Balanced, and to the Point
 This product, when used as directed, produces an industrial bleach that can cause serious harm.
This product, when used as directed, produces an industrial bleach that can cause serious harm.


